
The U.S. cannabis and hemp industry currently lacks comprehensive product characterization, primarily due to limited state testing regulations in this area and a lack of widespread implementation of current Good Manufacturing Practices (cGMP). While most states focus on testing a few active molecules, namely Δ9-THC, Δ9-THCA, CBD, and CBD-A, full identification of product components is generally not required. Furthermore, the imperfect definition of hemp in the 2018 Farm Bill has led to hemp businesses engaging in the synthesis of cannabinoids, such as Δ8-THC, from CBD with inadequate regulatory oversight. These chemical reactions involve harsh acids, metal catalysts, heat and/or total synthesis to convert CBD into Δ8-THC, hexahydrocannabinol (HHC), and other cannabinoids that are either not found in the plant in significant quantities or produced by the plant at all. The surge of products containing these novel cannabinoids has resulted in numerous adverse event reports received by the FDA, leading the FDA to issue a public warning about the serious health risks of consuming as Δ8-THC products.[1]
To help address the knowledge gap of impurities in hemp-derived products, Dr. Mahmoud ElSohly and his research team at the National Center for Natural Products Research, University of Mississippi, conducted a recent study published in the Journal of Natural Products. Their research aimed to isolate and characterize impurities present in a commercially available Δ8-THC distillate, uncovering previously unidentified impurities. Gas chromatography-mass spectrometry (GC-MS) was initially used to identify the distillate’s components, followed by liquid chromatography-mass spectrometry (LC-MS) to purify eleven of the impurities. The impurities were then characterized using various nuclear magnetic resonance (NMR) techniques to determine their chemical structures.
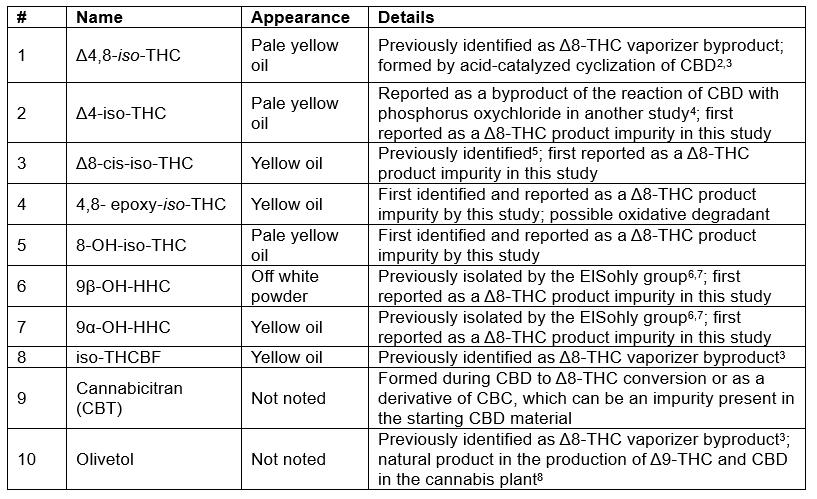
The ElSohly lab was able to identify most of the impurities by comparing the NMR data to the literature. However, several impurities were previously unreported in Δ8-THC products, and their pharmacological and toxicological effects remain largely unknown. In Table 1, we summarize key information about ten of the impurities isolated in this study. Additionally, the distillate contained other cannabinoids, including Δ9-THC, CBD, CBN, HHC, and Δ8-tetrahydrocannabivarin (Δ8-THCV). The next step is to isolate enough of these molecules to 1) use them as reference standards to determine their concentration in other commercially available products and to 2) investigate their toxicity and pharmacology profiles. Hopefully, through the continued work of researchers like those in the ElSohly lab, we will soon have answers to the following questions.
[1] 5 Things to Know about Delta-8 Tetrahydrocannabinol – Delta-8-THC. FDA. https://www.fda.gov/consumers/consumer-updates/5-things-know-about-delta-8-tetrahydrocannabinol-delta-8-thc
[2] Marzullo, P.; Foschi, F.; Coppini, D. A.; Fanchini, F.; Magnani, L.; Rusconi, S.; Luzzani, M.; Passarella, D. J. Nat. Prod. 2020, 83, 2894−2901
[3] Meehan-Atrash, J.; Rahman, I. Chem. Res. Toxicol. 2022, 35, 73−76
[4] Nalli, Y.; Jan, S.; Lauro, G.; Ur Rasool, J.; Lone, W. I.; Sarkar, A. R.; Banday, J.; Bifulco, G.; Laatsch, H.; Syed, S. H.; Ali, A.; et al. Nat. Prod. Res. 2021, 35, 471−480
[5] Terlouw, J.; Heerma, W.; Burgers, P.; Dijkstra, G.; Boon, A.; Kramer, H.; Salemink, C. Tetrahedron 1974, 30, 4243−4248.
[6] Ahmed, S. A.; Ross, S. A.; Slade, D.; Radwan, M. M.; Khan, I. A.; ElSohly, M. A. Phytochemistry 2015, 117, 194−199.
[7] Reggio, P. H.; McGaughey, G.; Odear, D. F.; Seltzman, H. H.; Compton, D. R.; Martin, B. R. Pharmacol., Biochem. Behav. 1991, 40, 479−486.
[8] Kearsey, L.J., Prandi, N., Karuppiah, V., Yan, C., Leys, D., Toogood, H., Takano, E., and Scrutton, N.S. FEBS J. 2020, 287(8), 1511-1524.
[9] Radwan, M. M., Wanas, A. S., Gul, W., Ibrahim, E. A., & ElSohly, M. A. (2023). Isolation and Characterization of Impurities in Commercially Marketed Δ 8 -THC Products. Journal of Natural Products, 86(4), 822–829. https://doi.org/10.1021/acs.